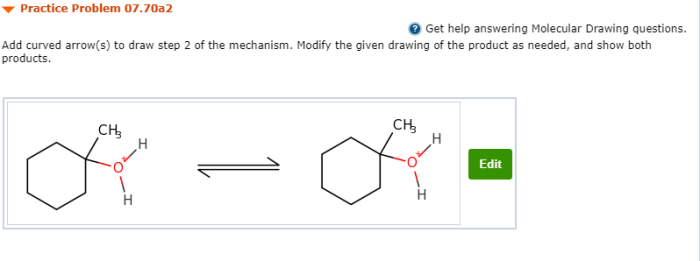
Add curved arrows to draw step 2 of the mechanism – In the realm of organic chemistry, the concept of curved arrows holds immense significance, serving as a visual representation of electron movement within chemical reactions. This article delves into the intricacies of adding curved arrows to depict step 2 of a reaction mechanism, shedding light on the transformation that occurs and the electron flow involved.
1. Curved Arrow Notation
Curved arrows are a graphical representation of electron movement in organic chemistry. They indicate the flow of electrons from one atom or molecule to another.
The head of the arrow points towards the atom or molecule that is gaining electrons, while the tail of the arrow points towards the atom or molecule that is losing electrons.
Examples of Curved Arrow Notation, Add curved arrows to draw step 2 of the mechanism
- Nucleophilic attack:A nucleophile attacks an electrophile, donating a pair of electrons to form a new bond.
- Electrophilic addition:An electrophile adds to a double or triple bond, forming a new bond between the electrophile and each of the carbons in the double or triple bond.
- Rearrangement:A rearrangement occurs when the atoms in a molecule rearrange to form a new molecule.
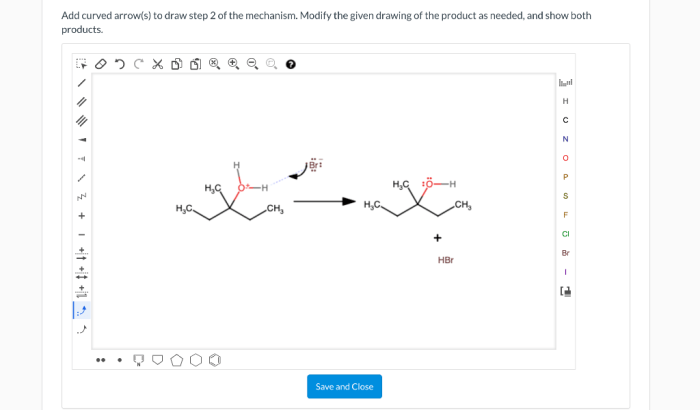
2. Step 2 of the Mechanism: Add Curved Arrows To Draw Step 2 Of The Mechanism
In step 2 of the mechanism, the nucleophile attacks the electrophile, donating a pair of electrons to form a new bond.
The curved arrow notation for this step is shown below:
The curved arrow shows the flow of electrons from the nucleophile to the electrophile, forming a new bond between the two atoms.
3. Electron Flow
The electron flow in step 2 of the mechanism is as follows:
- The nucleophile donates a pair of electrons to the electrophile.
- The electrophile accepts the pair of electrons, forming a new bond between the two atoms.
- The nucleophile becomes negatively charged, while the electrophile becomes positively charged.
4. Resonance Structures
In some cases, resonance structures may be involved in step 2 of the mechanism.
Resonance structures are different Lewis structures that represent the same molecule. They are connected by double-headed arrows.
Curved arrows can be used to show the interconversion of resonance structures.
5. Stereochemistry

In some cases, stereochemical changes may occur in step 2 of the mechanism.
Stereochemical changes are changes in the spatial arrangement of atoms in a molecule.
Curved arrows can be used to represent stereochemical changes.
6. Overall Mechanism
Step 2 of the mechanism is part of the overall mechanism of the reaction.
The overall mechanism shows all of the steps in the reaction, from the starting materials to the products.
The curved arrows in step 2 show how the electrons flow in this step.
FAQ Summary
What is the significance of curved arrows in organic chemistry?
Curved arrows depict the movement of electrons within a chemical reaction, providing insights into the transformation that occurs.
How do curved arrows represent electron flow?
The direction of the curved arrow indicates the path of electron movement, from the electron source (nucleophile) to the electron acceptor (electrophile).
What is the role of curved arrows in step 2 of a reaction mechanism?
In step 2, curved arrows illustrate the electron flow that leads to the formation of the product, highlighting the nucleophile and electrophile involved.