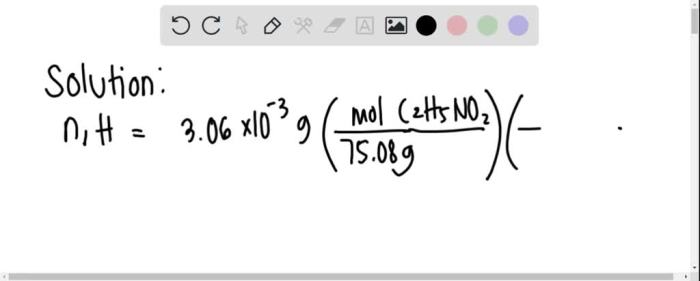
Determine the quantity of moles of hydrogen in 3.06 – Determining the quantity of moles of hydrogen in 3.06 grams is a fundamental task in chemistry, providing insights into the composition and behavior of substances. This exploration delves into the concept of moles, their significance in quantifying substances, and the steps involved in calculating the number of moles of hydrogen in a given mass.
Understanding the Mole Concept: Determine The Quantity Of Moles Of Hydrogen In 3.06
In chemistry, a mole is defined as the amount of substance that contains exactly 6.02214076 x 10 23elementary entities (atoms, molecules, ions, or electrons). This number is known as Avogadro’s number.
Moles are used to measure quantities of substances in a convenient and precise way. For example, one mole of water contains 6.02214076 x 10 23water molecules, and one mole of sodium chloride contains 6.02214076 x 10 23sodium ions and 6.02214076 x 10 23chloride ions.
Calculating the Quantity of Moles
The number of moles of a substance can be calculated using the following formula:
Number of moles = Mass (in grams) / Molar mass (in grams per mole)
For example, to determine the quantity of moles of hydrogen in 3.06 grams of hydrogen, we would use the following steps:
- Find the molar mass of hydrogen: 1.008 grams per mole
- Divide the mass of hydrogen by the molar mass of hydrogen: 3.06 grams / 1.008 grams per mole = 3.036 moles
Determining the Mass of Hydrogen, Determine the quantity of moles of hydrogen in 3.06
The mass of a substance can be calculated using the following formula:
Mass (in grams) = Number of moles x Molar mass (in grams per mole)
For example, to determine the mass of hydrogen in 3.06 moles of hydrogen, we would use the following steps:
- Multiply the number of moles of hydrogen by the molar mass of hydrogen: 3.06 moles x 1.008 grams per mole = 3.087 grams
Chemical Reactions Involving Hydrogen
Hydrogen is a highly reactive element that plays a vital role in many chemical reactions. Some examples of chemical reactions that involve hydrogen include:
- Combustion reactions: Hydrogen reacts with oxygen to produce water.
- Acid-base reactions: Hydrogen ions (H +) are produced when acids dissolve in water.
- Reduction-oxidation reactions: Hydrogen can be oxidized to form hydrogen ions or reduced to form hydrogen gas.
FAQ
What is the mole concept?
A mole is a unit of measurement in chemistry that represents a specific quantity of a substance. It is defined as the amount of substance that contains as many elementary entities (atoms, molecules, ions, or electrons) as there are atoms in 12 grams of carbon-12.
How do I calculate the number of moles of hydrogen in 3.06 grams?
To calculate the number of moles of hydrogen in 3.06 grams, you can use the formula: moles = mass (in grams) / molar mass. The molar mass of hydrogen is 1.008 grams per mole. So, moles = 3.06 grams / 1.008 grams per mole = 3.037 moles.