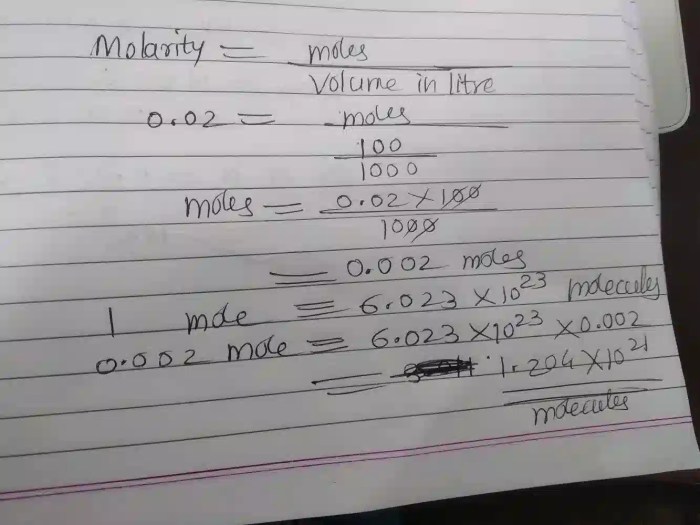How many molecules are there in 230 grams of CoCl2? This question embarks us on a scientific expedition into the realm of chemistry, where we unravel the intricate relationship between mass, moles, and the enigmatic world of molecules. Join us as we delve into the fascinating world of molecular quantification.
CoCl2, also known as cobalt(II) chloride, is a captivating compound that plays a significant role in various chemical processes. Its unique properties and applications have made it a subject of extensive research. In this discourse, we embark on a quest to determine the number of molecules present in a specific mass of CoCl2, providing a comprehensive understanding of its molecular composition.
How Many Molecules Are There in 230 Grams of CoCl2?

This article explores the concept of molar mass, moles, and Avogadro’s number to determine the number of molecules present in 230 grams of cobalt(II) chloride (CoCl2).
1. Molar Mass of CoCl2
Molar mass is a fundamental concept in chemistry, representing the mass of one mole of a substance. The molar mass of CoCl2 is the sum of the atomic masses of cobalt (Co) and two chlorine (Cl) atoms:
Molar Mass of CoCl2 = Atomic Mass of Co + 2 × Atomic Mass of Cl
Using the periodic table, we find:
- Atomic Mass of Co = 58.9332 g/mol
- Atomic Mass of Cl = 35.453 g/mol
Therefore, the molar mass of CoCl2 is:
Molar Mass of CoCl2 = 58.9332 g/mol + 2 × 35.453 g/mol = 129.840 g/mol
2. Number of Moles in 230 Grams of CoCl2
A mole is the SI unit of amount, representing a specific number of entities (atoms, molecules, ions, etc.). To determine the number of moles in 230 grams of CoCl2, we use the following formula:
Number of Moles = Mass of Substance / Molar Mass
Substituting the given values:
Number of Moles = 230 g / 129.840 g/mol = 1.772 moles
3. Avogadro’s Number and Molecular Count
Avogadro’s number, represented by NA, is a constant value approximately equal to 6.022 × 10 23entities per mole. It provides a bridge between the macroscopic and microscopic scales, allowing us to relate the number of moles to the number of molecules.
Using Avogadro’s number, we can calculate the number of molecules in 230 grams of CoCl2:
Number of Molecules = Number of Moles × Avogadro’s Number
Substituting the calculated number of moles:
Number of Molecules = 1.772 moles × 6.022 × 1023molecules/mole = 1.066 × 10 24molecules
4. Chemical Structure and Molecular Formula, How many molecules are there in 230 grams of cocl2
CoCl2, cobalt(II) chloride, has a simple chemical structure. It consists of a central cobalt ion (Co 2+) surrounded by two chloride ions (Cl –). The molecular formula, CoCl2, represents the stoichiometric ratio of the elements in the compound.
The molecular formula is crucial for determining the number of molecules present in a given mass of a substance. In this case, the molecular formula CoCl2 indicates that there is one molecule of CoCl2 for every mole of the substance.
FAQ Section: How Many Molecules Are There In 230 Grams Of Cocl2
What is the significance of molar mass in chemistry?
Molar mass serves as a fundamental property of a substance, representing the mass of one mole of that substance. It plays a pivotal role in stoichiometric calculations, enabling chemists to determine the quantitative relationships between reactants and products in chemical reactions.
How does Avogadro’s number contribute to understanding molecular count?
Avogadro’s number, a cornerstone of chemistry, represents the number of entities (atoms, molecules, ions, or electrons) present in one mole of a substance. It establishes a direct link between the macroscopic and microscopic scales, allowing scientists to bridge the gap between the mass and the number of molecules in a given sample.